If you or anyone you know needs to take heart disease medication then you should absolutely check their prescription. That’s because on Friday (July 6), Hong Kong’s Department of Health issued a recall for five heart disease prescription drugs that can cause cancer.
Source: hong kong government
Based on a report by SCMP, the drugs contain valsartan which are given to patients to treat hypertension and heart failure. Actavis, a pharmaceutical company had outsourced the production of the drug to a company in mainland China, Zhejiang Huahai.
However, they issued a recall of the medicine after it was discovered that a Chinese factory was found to produce medicine containing a carcinogenic substance. The substance is known as N-nitrosodimethylamine (NDMA) and has been identified as a probable carcinogen in humans.
Source: hong kong government
The list of prescription drugs affected are namely:
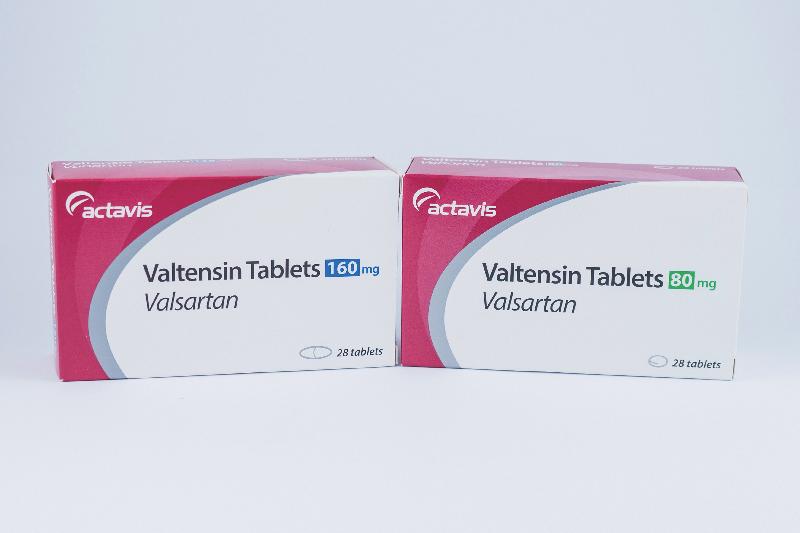
- Valtensin 160mg tablets
- Valtensin 80mg tablets
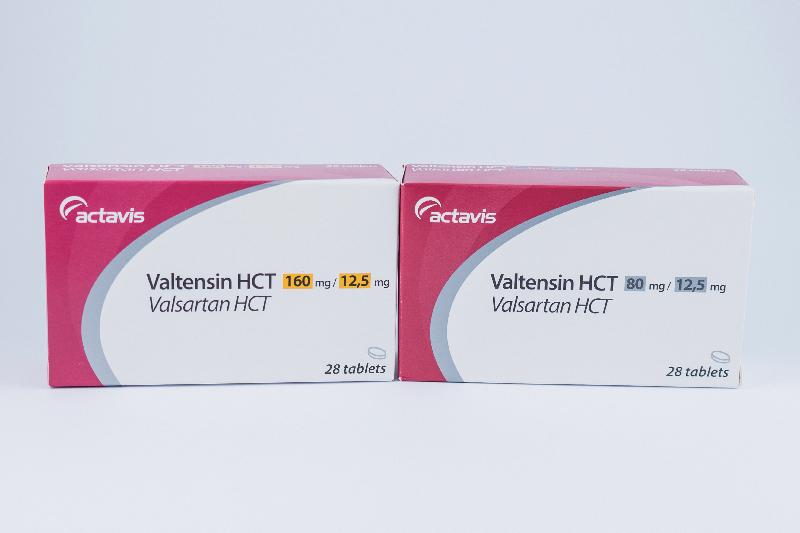
- Valtensin HCT tablets 160/12.5mg
- Valtensin HCT tablets 80/12.5mg
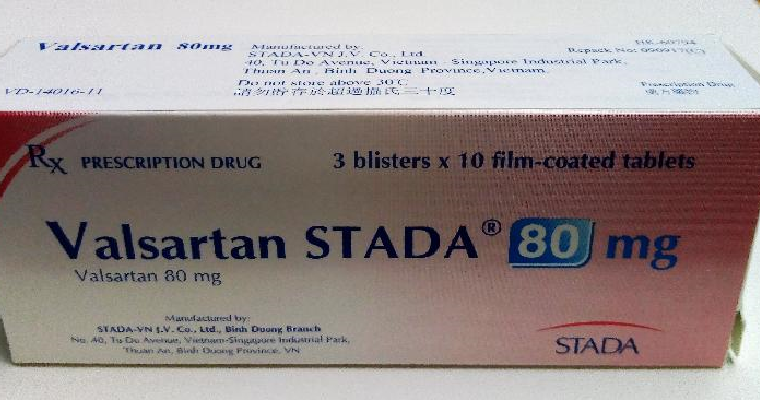
- Valsartan Stada 80mg tablets
Doctors in Hong Kong have been advised to stop prescribing the medicine which affects about 30,000 patients.
The Hong Kong health department advised patients to continue taking the pills but urged them to consult their doctor if they wanted to change the prescription. It is not advisable for them to stop taking the medicine suddenly as this could lead to uncontrolled high blood pressure that can be fatal.
Source: hong kong government
Cardiologist Dr Bernard Wong Bun-lap said, “The chance of contracting cancer is related the amount of carcinogen, as well as the patients’ physical condition. Patients are advised to use other medicines under doctors’ instructions no matter how much NDMA it takes to give them cancer.”
This problem does not affect only Hong Kong but there are batches of valsartan drugs that have been shipped to the European Union where they are also recalling the medicine. The medicine in question is used widely in public hospitals as they are cheaper versions.
Luckily for Malaysians, the Director-General of Health Malaysia has issued a press statement, saying that these Valtensin medicines were not registered in Malaysia which means they are not allowed to be sold in Malaysia. He advises everyone to only buy medicines that are lawfully registered in Malaysia to ensure the safety of the product. Stay away from these pills at the moment!
UPDATE: Malaysians would be glad to know that the Valsartan products sold here are NOT affected by the product recall. These products include Diovan®, Co-Diovan®, Exforge®, Exforge HCT® and Entresto® and the manufacturer, Novartis has confirmed that Malaysia is not impacted as they use a different active product ingredient source.
The 23 countries that were affected are Germany, Norway, Finland, Sweden, Hungary, Netherland, Austria, Ireland, Bulgaria, Italy, Spain, Portugal, Belgium, Luxemburg, France, Poland, Croatia, Lithuania, Greece, Canada, Bosnia and Herzegovina, Bahrain and Malta.
In a media statement, Novartis said that they are “committed to ensuring that all our marketed products meet the highest quality standards.” They also advise customers who are using these products to consult a physician if they wish to stop using the medicines as it could be detrimental to their health.
Consult your doctor if you’re unsure because it is better to be safe than sorry!
Also read: Makeup From These 8 Korean Brands Are Being Recalled For Having Harmful Metal Elements