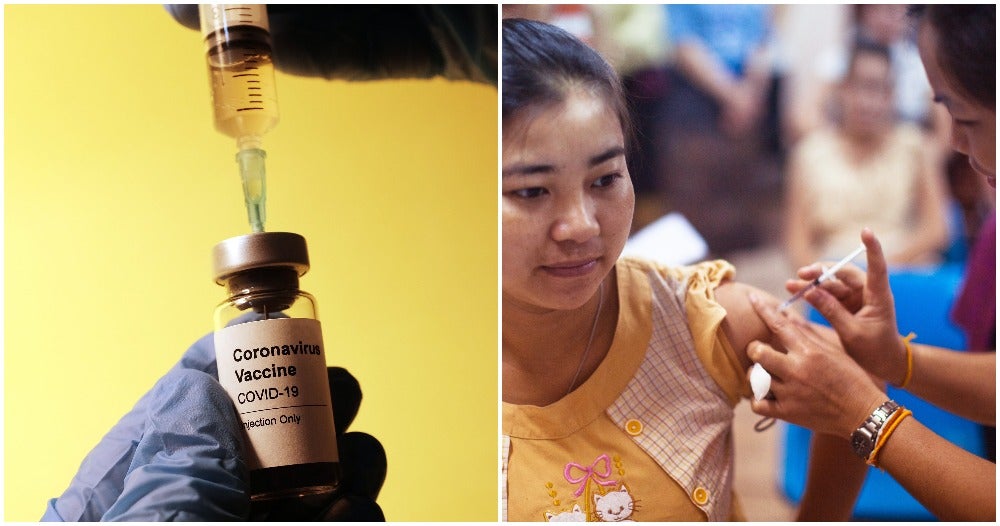
In December 2020, the Ministry of Health (MOH) conducted a survey to see whether Malaysians are willing to get vaccinated for Covid-19.
The survey was done from 21 -28 December and it was participated by 212,000 Malaysians, reported MOH. 67% of the respondents are willing to take the vaccine as a big majority is confident that the vaccine will work. However, 17% of the respondents were unsure as they are worried about the effectiveness of the vaccine. 16% did not want to get vaccinated.

The government seeks to vaccinate 70% of the population to create herd immunity (23 million people) and they will start by vaccinating:
- The frontliners
- People who are at high risk (have chronic illnesses) and elderly above 60 years old
- People above the age of 18
The health minister has also said that the registration for the vaccine will be open soon. Minister of Science, Technology and Innovation of Malaysia, Khairy Jamaluddin has said that registration for the vaccine can be done through MySejahtera when the time comes.
The main vaccine that will be used is the Pfizer-BioNTech vaccine which will arrive on our shores at the end of February. It has also been granted conditional registration for use in Malaysia by the National Pharmaceutical Regulatory Agency NPRA.
I was just informed by @DGHisham that NPRA has given conditional registration for the Pfizer vaccine. We are still waiting for a few additional info from Pfizer but this means it can be used in Malaysia. Congratulations NPRA on the quick registration.
— Khairy Jamaluddin ??? (@Khairykj) January 8, 2021
However, our health Director-General has said that the government is looking at different vaccines.
Kerajaan telah & sedang menjalankan rundingan & tindakan “preemptive” utk mendapatkan vaksin dari pelbagai pihak & cara. NPRA menyusuli & memberi keutamaan dlm menilai keberkesanan & keselamatan vaksin. Tempoh biasa diambil 90-120 hari tetapi sasaran KKM adalah kurang drp 90 hari pic.twitter.com/cvjsutSZKF
— Noor Hisham Abdullah (@DGHisham) December 22, 2020
Multiple countries have different experiences with the usage of Sinovac. Turkey reported that it is 91.25% effective while Indonesia said that the vaccine is 65.3% effective. Brazil reports that the vaccine is only 50.4% effective.
Speaking on this, KJ stresses: “If we are not satisfied with the safety and efficacy, we will not go through with the procurement. We will review all data and make a decision.”
Our vaccine procurement is subject to NPRA @KKMPutrajaya approval. If we are not satisfied with the safety and efficacy, we will not go through with the procurement. Sinovac’s clinical data is just being released. We will review the data and decide. https://t.co/gWxlWVnbWM
— Khairy Jamaluddin ??? (@Khairykj) January 13, 2021
The ministry has also stated that as of now, vaccines are effective against the coronavirus even if there are mutations. Be that as it may, the ministry will monitor the development of the mutations.
Speaking on the side effects of Covid-19 vaccination, they have stated that there will be minimal side effects which include pain in the vaccination area, fatigue, headache, and fever. These side effects can be resolved by taking paracetamol before getting the vaccination.

Will you be registering for the vaccination once it’s out? Let us know.
Also read: Video: Doctor Couldn’t Stop Trembling While Giving President Jokowi His Covid-19 Vaccine