Based on a large-scale trial of their Covid-19 vaccine booster shots, Pfizer and BioNTech have announced on Thursday (21 October) that their booster shots show promising results restoring full protection against the disease.
As reported by BERNAMA, in a Phase 3 randomised, controlled trial, a Pfizer-BioNTech booster dose was administered to more than 10,000 individuals 16 years of age and older, who previously received the Pfizer-BioNTech primary two-dose series.
The trial showed relative vaccine efficacy of 95.6 per cent compared to those who did not receive any boosters. Albert Bourla, Pfizer chairman and CEO said,
“These results provide further evidence of the benefits of boosters as we aim to keep people well-protected against this disease.”
In September, US FDA has allowed booster shots for the Pfizer-BioNTech Covid-19 vaccine, authorizing a single booster dose of the Pfizer-BioNTech vaccine which can be administered at least 6 months after completion of the primary series to individuals 65 years of age and older, and 18 through 64 years of age with frequent institutional or occupational exposure to SARS-CoV-2.
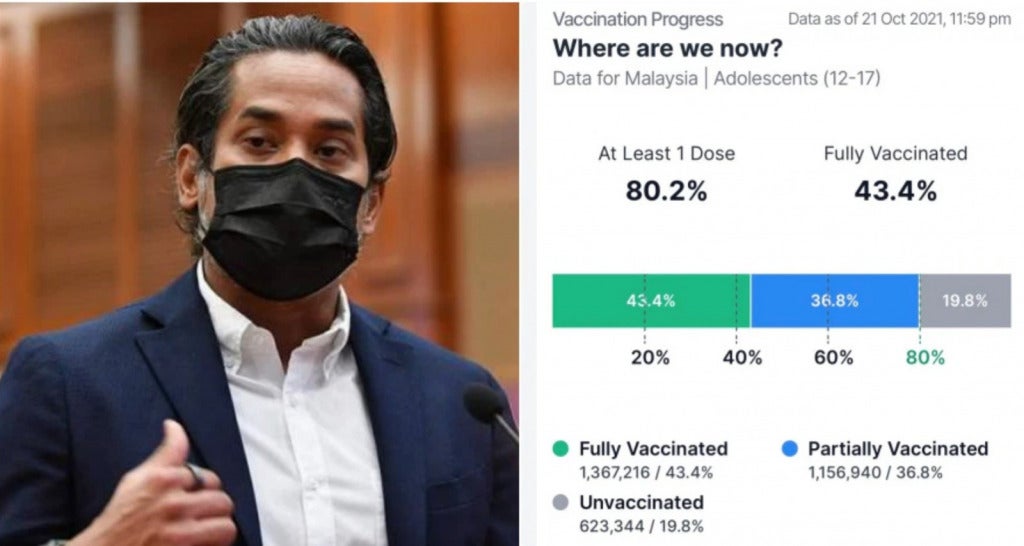
Also read: KJ: 80% Of Adolescents In M’sia Have Received 1 Dose Of The Vaccine In Less Than 2 Months