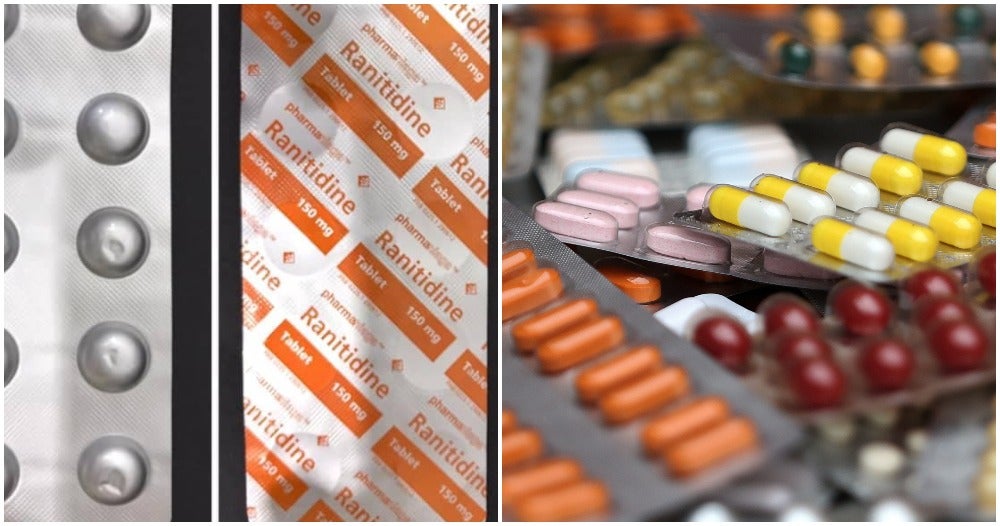
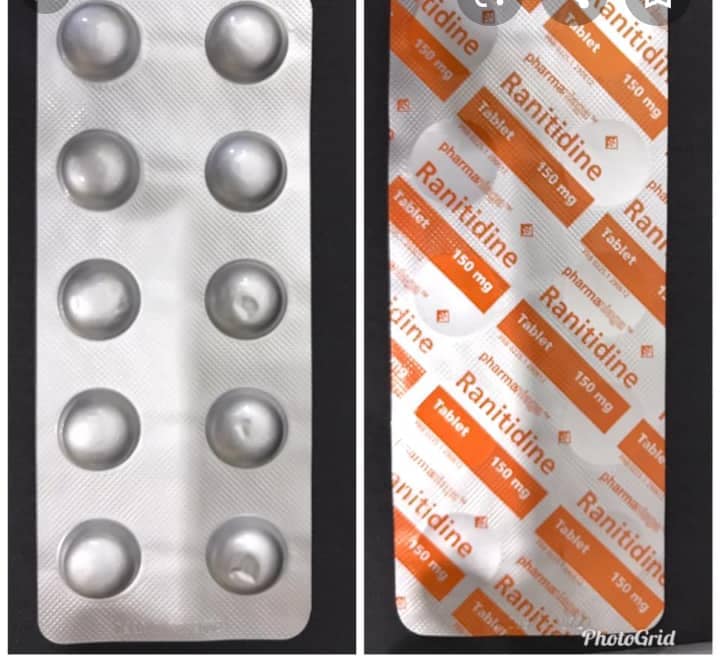
The Ministry of Health is recalling 10 medications that have Ranitidine in them, reported Sinar Harian.
Ranitidine is a type of medication that helps to treat heartburn and also gastric problems by reducing the rate of acid production in the stomach.
Source: fscebook
Datuk Dr Noor Hisham Abdullah, the Director General of the Ministry of Health, said that the products contain a higher than the recommended amount of an impurity known as N-Nitrosodimethylamine (NDMA). He confirmed the findings after looking at the results from the lab tests ran by the ministry and parties involved.
“NDMA is an impurity that can be found in low levels in certain foods and drinks, air pollutant and a result of certain factory processes.”
According to the International Agency for Research on Cancer (IARC), NDMA can increase the risk of cancer if the user takes it in the long term.
Affected products are as follows:
- Zantac Injection 50mg/2ml with the registration number MAL19950591ARZ
- Zantac Syrup 150mg/10ml (MAL19910829ACRZ)
- Zantac Tablet 150mg (Not marketed in Malaysia) (MAL19871471ARZ)
- Hyzan Tablet 150mg (MAL19984063AZ)
- Vesyca Film Coated Tablet 150mg (MAL19991131AZ).
- Apo-Ranitidine 300mg Tablet (MAL19988448AZ)
- Apo-Ranitidine 150mg Tablet (MAL19986966AZ)
- Ranitic Tablet 150mg (MAL19988460AZ)
- Pharmaniaga Ranitidine Tablet 150mg (MAL19987368AZ)
- Pharmaniaga Ranitidine Tablet 300mg (MAL19987370AZ)
However, he reminded individuals taking ranitidine that the benefits of continuing treatment were more than the risk potential of consuming small amounts of NDMA. He said that they could also get advice from health professionals if they wish to change to another medication.
Source: the star
Regulatory actions will be taken towards those products and the public will be informed about it from time to time.
That sounds really dangerous! If you have any of those medications, please do try to change it and opt for another!
Also read: 25yo Woman Self-Prescribed Meds For Her Migraines, Passes Away After 3 Days in Hospital